Biology of Childhood Leukemia
by Julia Vassey
Joe Wiemels, a molecular epidemiologist at the UC Berkeley Center for Integrative Research on Childhood Leukemia and the Environment (CIRCLE) has spent 30 years deciphering the complex biology of childhood leukemia – the most prevalent cancer in children.
Leading leukemia scientists, including CIRCLE researchers, have used observational studies to draw links between the disease and unhealthy lifestyles, aberrant immune development, genetic risk factors, and exposure to environmental chemicals. But the biological mechanisms underpinning childhood leukemia remain unclear.
Wiemels is on a quest to find harder evidence – drilling down to the molecular level to understand how these risk factors can alter the way our genes work and, ultimately, cause cancer.
He has been investigating the role of genetics in childhood leukemia since the 1990s, when he started his postdoctoral program at the University of London’s Institute of Cancer Research. There, he worked with his mentor Mel Greaves, a well-known cancer cell biologist and childhood leukemia researcher.
The two scientists and their colleagues were thinking about cancer non-stop – in the labs at work and after hours, too, even in the local pubs, where they found loads of punters drowning in beer, fish-n-chips, and cigarettes.
“Eating well is good for you! Smoking is bad for you!” Wiemels mimicked. “People get tired of hearing these headlines.”
He came to believe that what people want is to have more tangible proof that these habits can really affect their health, “something that is specific, something that they can hold on to, meaningful for me,” Wiemels says.
“Living in London was great fun and fruitful for research,” Wiemels says. His work there helped define the earliest genetic changes in a child’s life that lead to a leukemia diagnosis years later – these mutations occurred even before the child was born.
In the early 2000s, Wiemels moved to Northern California to start his own research lab, working alongside the UC Berkeley California Childhood Leukemia Study at CIRCLE and the Department of Epidemiology at the University of California, San Francisco.
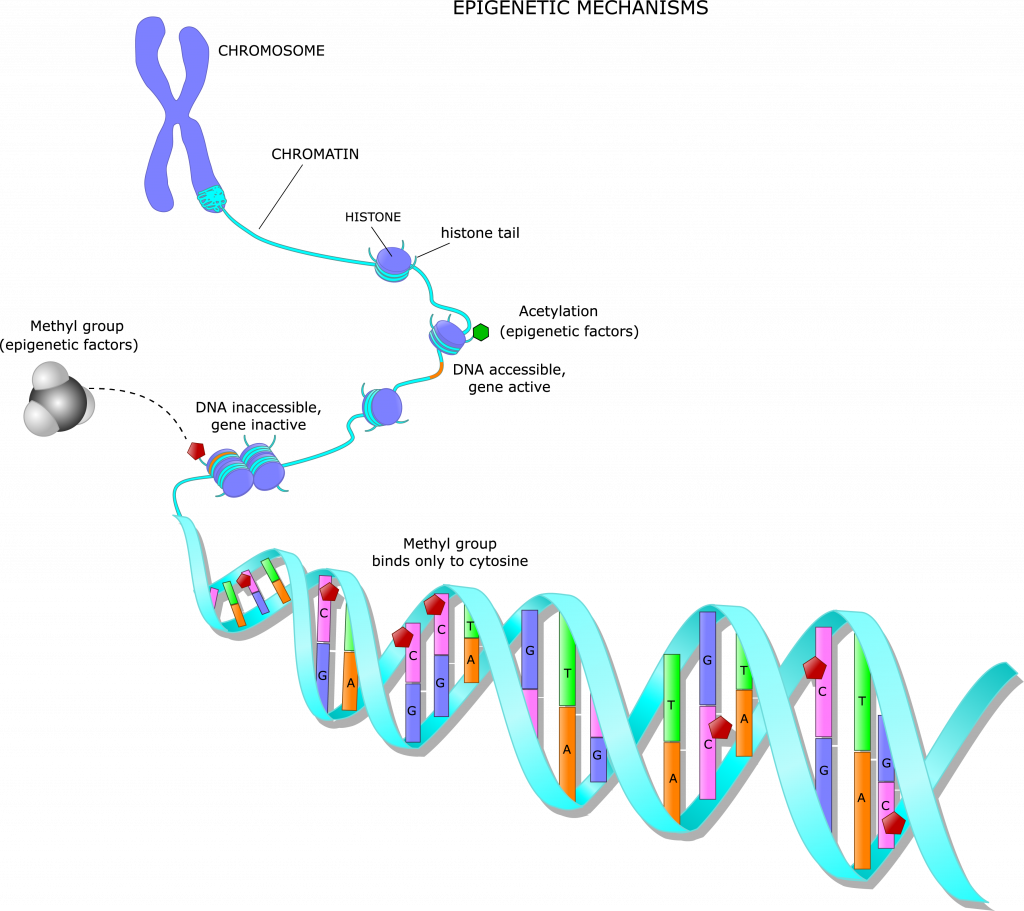
In search of indisputable evidence of what causes cancer, Wiemels jumped headlong into the innovative science of epigenetics – the study of cellular modifications to DNA or, literally, what’s “on top of” genetics.
Epigenetics and Leukemia Risk
Epigenetic changes do not alter the genetic code itself, but they affect the way genes behave in our bodies: deciding what proteins are produced and what cells and tissues are formed.
Epigenetics can be as influential as a great director on a movie set. Two identical actors may be reading from the same script, but the director’s notes on blocking, cadence, and emotion can change the whole scene. Similarly, two cells with the same DNA code can have vastly different manifestations, depending on the epigenetic modifications present.
Epigenetics can also help explain why people have certain physical or physiological differences like the color of the skin, sleep patterns or even food preferences.
Epigenetic modifications also affect our bodies’ interaction with the environment, nutrition and exercise or infections every single minute of our lives – from conception to death.
“Epigenetics is everywhere,” says Wiemels, looking at the sunlight coming through the window in his office. “Right here! This light is inducing epigenetic changes in my eyes and skin.”
Wiemels is focusing on a specific epigenetic mechanism called methylation, which adds methyl groups – consisting of a carbon atom and three hydrogens (CH3) – to certain parts of a DNA molecule. Methylation can turn our genes on or off, making them active or dormant.
“If you are a liver, you don’t want to be a bone.” That was Wiemels pithy explanation.
In other words, each cell in our body has a specific purpose – one that is dictated by DNA methylation. Early in its life, each liver cell must “turn off” the genes that would have it making proteins to form bones. Likewise, each bone cell would amplify those genes and silence the ones that define a liver cell.
But in certain diseases like cancer, genes get switched away from a healthy state.

Childhood leukemia stems from a malfunction of white blood cells – B cells, which patrol the body like watchmen looking for intruding viruses and bacteria that would otherwise weaken the immune system.
When leukemia strikes, it’s because the bone marrow is producing unhealthy, immature B cells, which have too many or too few methyl groups attached to their DNA, along with certain genetic mutations in the DNA. This abnormal DNA methylation throws the immune system into disarray.
Wiemels showed that children diagnosed with leukemia had abnormal DNA methylation across about 10 percent of the genome in their white B cells. That’s a lot!
Wiemels believes many of these drastic epigenetic changes can be linked to our adaptations to environmental factors, like maternal cigarette smoking during pregnancy, or responding to infectious exposures.
The Smoking Gun
Smoking has already been shown to exert a strong effect on DNA methylation, even more so than other environmental chemicals CIRCLE is investigating, according to Wiemels.


Wiemels and his colleague Adam de Smith, a researcher at the University of Southern California, Los Angeles, discovered that leukemia patients whose mothers smoked during pregnancy had tumors with more gene deletions – a chunk of DNA that is missing from a chromosome.
For each additional five cigarettes smoked daily during pregnancy, there was a 22 percent increase in the number of deletions; and for each additional five cigarettes smoked daily during breastfeeding, there was a 74 percent increase in the number of deletions.
While the results of previous research aimed to find the association between maternal smoking and the risk of leukemia were inconsistent, this was the first study to link maternal smoking with a specific mutation in childhood leukemia.
“This finding provides a rare glimpse at a causal factor, smoking, that creates a specific, definable wound in DNA. It’s like looking at a gunshot bullet hole,” Wiemels says.
The deletions have also been blamed on triggering a gene re-arrangement mechanism called “recombinase activating gene” – an exchange of genetic material within chromosomes. While this process is common and occurs naturally in lymphocytes (B and T cells), it can become detrimental for health, if stimulated by environmental chemicals like smoking.
By looking at epigenetic changes within smoking-sensitive regions of the DNA as well as the recombinase activating gene-associated deletions, Wiemels hopes to pinpoint how exactly tobacco caused the mutations.
To achieve his vision, Wiemels with other researchers from CIRCLE and the University of Southern California Center for Genetic Epidemiology that he has recently joined, will examine blood samples collected from 8,000 children, half of whom have been diagnosed with leukemia. The experiment is part of a new project funded by California’s Tobacco-Related Disease Research Program. Wiemels’ laboratory will use an innovative biomarker to determine which children were exposed to tobacco in utero.
“We will examine neonatal blood that provides the history of maternal smoking during pregnancy. We would be able to say if smoking was occurring and whether it was a little or a lot using this sensitive biological marker,” says Wiemels.
The researchers want to understand why some children who were exposed to tobacco in utero end up getting leukemia while others do not.
“The result from this analysis will directly address cancer prevention and help identify those individuals who would be especially susceptible to tobacco exposure,” Wiemels says.
In the future, Wiemels plans to investigate tobacco-caused epigenetic changes in sperm of the fathers who smoke, to follow-up on CIRCLE’s earlier findings that showed a link between paternal preconception smoking and the risk for childhood leukemia.
CMV, the Silent Killer
The role of infections in childhood leukemia is another area of Wiemels’ research.
In the 1980s and later on, his U.K mentor Greaves made a series of discoveries showing that mild infections early in life – the ones that children often catch at daycare centers – help build the immune system and reduce the risk of leukemia.
Wiemels is focusing on cytomegalovirus or “CMV” – a common virus to which many people are exposed (often without knowing it). CMV can remain dormant in a healthy human body for person’s whole lifetime, but can bloom into a fulminant infection among individuals whose immune systems are weakened or compromised, for example, by HIV or after organ transplantation. Wiemels hypothesizes that the virus can become dangerous only when a mother passes it to a child while still in the womb, subsequently increasing the risk of leukemia.
Previous CIRCLE studies found that children who developed leukemia were about three times more likely to have had the CMV virus at birth compared to healthy control children who did not get the cancer at that age. This may be a particular big problem for Latino children, who are more likely to be diagnosed with leukemia than other groups.
“The frequency of passing CMV from a mom to a child prenatally is a little bit higher in Hispanics, which may partially explain the higher risk of leukemia observed within this group – something we want to follow up on,” Wiemels says.
Folate Helps
Early pregnancy is a critical moment for epigenetic programming. It’s “when the most DNA methylation patterns are set up all over the body for every single tissue,” Wiemels explains.
As such, factors that impact DNA methylation during pregnancy can be strong risk factors for childhood leukemia.
Folate is the main shuttle that carries methyl groups around the body during cellular development, delivering them to specific DNA sites that needs to be methylated.
If a child growing inside the mother’s womb doesn’t get enough folate, it may lead to unhealthy epigenetic changes.


These alterations can be carried on to birth and early childhood to manifest as a leukemia around the age of two or three, when kids most contract the disease. Wiemels and his colleagues have observed such changes in children who received less folate from their mothers in early pregnancy, compared to mothers with better nutrition, and are working to understand how these changes may affect leukemia risk.
What’s Next
Wiemels is committed to his quest for hard evidence of what causes childhood leukemia, but he admits that epigenetics is quite challenging to study.
He calls it “a moving target,” because of the constant changes that occur in a cell’s composition during the first weeks of a child’s life. For example, the number of erythrocytes – the red blood cells – naturally decline in an infant’s body soon after birth. Because each type of blood cell has a unique level of DNA methylation, as the blood’s composition changes, so too does the DNA methylation that Wiemels and his team measures.
“How do you control for all these factors and still get meaningful results in the end for the individual? Wiemels says. “A lot of epidemiologists may point fingers at me saying that you’ll never going to prove anything.”
But Wiemels hopes epigenetic discoveries will eventually “become the lexicon of cancer prevention,” Wiemels says.
On a recent visit to his old laboratory in London, Dr. Wiemels had this discussion with his former mentor.
“Greaves is 77, but he is still energetic and full of command,” Wiemels says. Greaves hopes to make new discoveries in childhood leukemia research by taking a closer look at a microbiome – a vast army of microbes that protects infants in the wombs and soon after birth from harmful infections that can trigger cancer.
Wiemels believes the recipe for success in fighting leukemia is to gather all possible data at different stages of a child and his parents’ lives, learning about the child’s birth, genes, and exposures to infections and chemicals like pesticides.
It is also important to look at all the factors in combination, instead of analyzing it one a time. Powerful statistical tools and DNA sequencing methods that have recently emerged allow to study “hundreds and thousands to millions of data points,” Wiemels says.
“Following these steps, I hope we are going to see that there are impacts of pesticides on some children that may not be evident for other children. Or we may discover that kids have different susceptibility to infections. This will show us a clear causal route to leukemia for each person with the disease and point towards early detection and prevention,” he says.
Comparing different types of cancers may also bring new insights. In his new researcher position at the University of Southern California Center for Genetic Epidemiology, Wiemels is focused on brain tumors’ origin and prevention, while continuing investigating the causes of leukemia at CIRCLE.
Wiemels will examine pediatric glioma brain tumors with a similar type of analysis that was done by the CIRCLE researchers when they looked at the environmental risk factors for leukemia.
All childhood cancers, including glioma and leukemia, are similar. Compared to adult cancers, they are genetically simple, they develop quickly and occur in tissues that are rapidly emerging and growing, making children particularly susceptible to outside disruptions – from chemicals, diet or infections.
But despite these similarities, each child has their own pathway to cancer, – and that is researchers’ greatest challenge to prevention, according to Wiemels.
“In this era of ‘personalized medicine’ we must also imagine ‘personalized prevention’ and create a society, in which each of us with our differences can thrive,” Wiemels says.


