Project 3: Prenatal Exposures, Constitutive Genetics, DNA Methylation & Childhood Leukemia
The Epigenetics project (Project 3) will investigate connections between prenatal chemical exposures, epigenetic modifications, and childhood leukemia risk. The Epigenetics Project is led by Dr. Joseph Wiemels, an expert in the molecular epidemiology of childhood cancers. Dr. Wiemels’ seminal papers on the natural history of childhood leukemia, demonstrated that the disease is often initiated in utero. As such, a major focus of CIRCLE is to discover the prenatal events that cause leukemia later in childhood. One hypothesis is that epigenetic modifications during development can predispose a child to leukemia.
What is epigenetics?
Epigenetics literally means “above” or “on top of” genetics. Epigenetics are modifications to the outer surface of a DNA molecule that turn gene expression “on” or “off.” Epigenetic modifications do not change the DNA sequence; rather, they affect how genes are transcribed into proteins. One example of an epigenetic change is DNA methylation — the addition of a methyl group to the DNA structure — which can alter the expression of nearby genes. Epigenetic modifications can have a very strong influence on phenotypes, indeed, the functional inactivation of a gene by DNA methylation can be as powerful as a gene mutation.


Two identical DNA sequences could produce vastly different phenotypes given divergent epigenetics.


If you think of our DNA as an immense piano keyboard and our genes as keys—each key symbolizing a segment of DNA responsible for a particular note, or trait, and all the keys combining to make us who we are—then epigenetic processes determine when and how each key can be struck, changing the tune being played.
-Peter Miller, National Geographic
Epigenetics and Childhood Leukemia
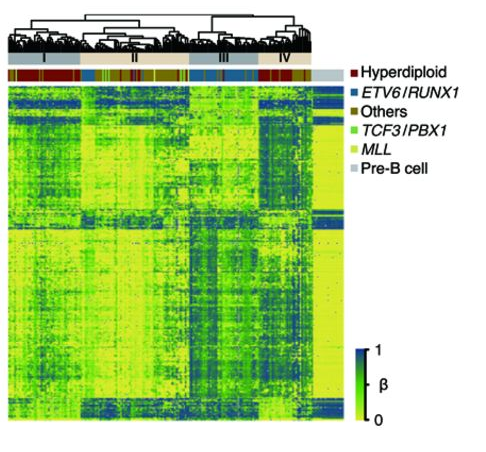
DNA methylation is critical during cell development so that genes which are not needed are permanently repressed in a cell that becomes specialized for a specific function. In cancer, DNA methylation processes can go awry; cancer cells are typically globally less methylated than their normal cell counterparts while having more DNA methylation in the control regions of key tumor suppressor genes. Like all cancers, childhood leukemia is characterized by DNA methylation changes in its target organ, blood. CIRCLE has reported that childhood leukemia tumor cells are profoundly altered from their pre-B cell precursors with regards to DNA methylation. Environmental risk factors for childhood leukemia, including polycyclic aromatic hydrocarbons and folic acid (protective), are also known to affect DNA methylation. CIRCLE is investigating the relationships between in utero environmental exposures, DNA methylation at birth, genetics, and childhood leukemia risk.
CIRCLE Findings